 |
| Presidente of USA, Jonh Biden and the Vice-President, Kamala Harris (imagem in https://www1.folha.uol.com.br/mundo/2021/05) |
Ficamos espantados, mas é mesmo assim: os Estados Unidos acabam de decretar o fim do uso da máscara e distanciamento entre as pessoas.
Quando será que Portugal poderá fazer o mesmo? Não há pressa, é preciso a maior segurança para o poder fazer e isso deve acontecer só quando as autoridades de saúde e o governo o determinarem! Não esqueçamos, nunca, esta regra, pois é para nosso bem e da nossa saúde.
Sobre o que a ciência nos pode dizer sobre isto, pesquisei e encontrei o seguinte:
em Science Brief: Background Rationale and Evidence for Public Health Recommendations for Fully Vaccinated People
Updated Apr. 2, 2021
Key Points
COVID-19 vaccines currently authorized in the United States are effective against COVID-19, including severe disease.
Preliminary evidence suggests that the currently authorized COVID-19 vaccines may provide some protection against a variety of strains, including B.1.1.7 (originally identified in the United Kingdom). Reduced antibody neutralization and efficacy have been observed for the B.1.351 strain (originally identified in South Africa). However, across studies, antibody neutralizing activity of sera from vaccinated people was still generally higher than that observed for convalescent sera from people who have recovered from COVID-19.
A growing body of evidence suggests that fully vaccinated people are less likely to have asymptomatic infection and potentially less likely to transmit SARS-CoV-2 to others. However, further investigation is ongoing.
Modeling studies suggest that preventive measures such as mask use and social distancing will continue to be important during vaccine implementation. However, there are ways to take a balanced approach by allowing vaccinated people to resume some lower-risk activities.
Taking steps towards relaxing certain measures for vaccinated people may help improve COVID-19 vaccine acceptance and uptake.
The risks of SARS-CoV-2 infection in fully vaccinated people cannot be completely eliminated as long as there is continued community transmission of the virus. Vaccinated people could potentially still get COVID-19 and spread it to others. However, the benefits of relaxing some measures such as testing and self-quarantine requirements for travelers, post-exposure quarantine requirements and reducing social isolation may outweigh the residual risk of fully vaccinated people becoming ill with COVID-19 or transmitting the virus to others.
At this time, there are limited data on vaccine protection in people who are immunocompromised. People with immunocompromising conditions, including those taking immunosuppressive medications, should discuss the need for personal protective measures after vaccination with their healthcare provider.
See updated guidance for fully vaccinated people including updated public health recommendations for domestic and international travel. This updated science brief reflects recent changes to the guidance document and will continue to be updated as more information becomes available.
Background
Comprehensive prevention measures are critical strategies to reduce the burden of SARS-CoV-2 in the United States. These prevention measures include wearing a mask, maintaining at least six feet of physical distance from others, avoiding crowds, avoiding poorly ventilated spaces, hand hygiene, cleaning and disinfection, following CDC travel guidance, and following workplace or school guidance related to personal protective equipment use or SARS-CoV-2 testing.1
COVID-19 vaccination is an additional critical prevention measure to help end the COVID-19 pandemic. There are currently three COVID-19 vaccines authorized by the Food and Drug Administration for emergency use: two mRNA vaccines (Pfizer-BioNTech, Moderna) and one viral vector vaccine (Janssen [Johnson & Johnson]). People are considered fully vaccinated if they are ≥2 weeks following receipt of the second dose in a 2-dose series (mRNA vaccines), or ≥2 weeks following receipt of a single-dose vaccine (Janssen).
While some prevention measures will continue to be necessary regardless of vaccination status, fully vaccinated people without immunocompromising conditions that may reduce their response to vaccination may be able to engage in some activities with low or reduced risk of acquiring or transmitting COVID-19. The benefits of avoiding disruptions such as unnecessary quarantine and social isolation may outweigh the residual risk of becoming ill with COVID-19 or transmitting the virus to others. The ability of vaccinated people to gradually resume some aspects of normal life will optimize well-being and may help improve vaccine acceptance.2
Public health recommendations for people fully vaccinated with COVID-19 vaccines must consider the evidence, including vaccine efficacy against symptomatic and asymptomatic COVID-19, as well as vaccine impact on SARS-CoV-2 transmission. However, other individual and societal factors are important when evaluating the benefits and potential harms of prevention measures among vaccinated individuals. The Advisory Committee on Immunization Practices and CDC routinely consider factors such as population values, acceptability, and feasibility of implementation when making vaccine recommendations.3 These considerations are also useful when making public health recommendations for fully vaccinated people.
In this scientific brief, we summarize evidence available through March 29, 2021, for the currently authorized COVID-19 vaccines (administered according to the recommended schedules) and additional considerations used to inform public health recommendations for fully vaccinated people, including:
Vaccine efficacy and effectiveness against SARS-CoV-2 infection
Vaccine performance against emerging SARS-CoV-2 variant strains
Impact of prevention measures in the context of vaccination
Population attitudes and behaviors towards vaccination and prevention measures
Rationale and evidence for travel associated public health recommendations for fully vaccinated travelers
COVID-19 vaccine efficacy and effectiveness
Vaccine efficacy refers to how well a vaccine performs in a carefully controlled clinical trial, whereas effectiveness describes its performance in the real world. Evidence demonstrates that the authorized COVID-19 vaccines are both efficacious and effective against symptomatic, laboratory-confirmed COVID-19, including severe forms of the disease. In addition, a growing body of evidence suggests that COVID-19 vaccines may also reduce asymptomatic infection, and potentially transmission. Substantial reductions in SARS-CoV-2 infections (both symptomatic and asymptomatic) will have the positive benefit of helping to reduce overall levels of disease, and therefore, transmission in the United States. However, further investigations are ongoing to assess the impact of COVID-19 vaccination on transmission.
Animal challenge studies
Rhesus macaque challenge studies provided the first evidence of the potential protective effects of Pfizer-BioNTech, Moderna, and Janssen COVID-19 vaccines against SARS-CoV-2 infection, including asymptomatic infection. Vaccinated macaques developed neutralizing antibodies that exceeded those in human convalescent sera and showed no or minimal signs of clinical disease after SARS-CoV-2 challenge 4-6. In addition, COVID-19 vaccination prevented or limited viral replication in the upper and lower respiratory tracts, which may have implications for transmission of the virus among humans 4-6.
Vaccine efficacy from human clinical trials
Clinical trials have demonstrated the authorized COVID-19 vaccines to be efficacious against laboratory-confirmed, symptomatic COVID-19, including severe forms of the disease, with evidence for protection against asymptomatic SARS-CoV-2 infection as well 7-13 (Box 1).
Box 1. Summary of vaccine efficacy estimates for authorized COVID-19 vaccines
All authorized COVID-19 vaccines demonstrated efficacy (range 65% to 95%) against symptomatic, laboratory-confirmed COVID-19.
For each authorized COVID-19 vaccine, the overall efficacy was similar to the efficacy across different populations, including elderly and younger adults, in people with and without underlying health conditions, and in people representing different races and ethnicities.
All authorized COVID-19 vaccines demonstrated high efficacy (≥89%) against COVID-19 severe enough to require hospitalization.
All authorized COVID-19 vaccines demonstrated high efficacy against COVID-19 associated death.
In the clinical trials, no participants who received a COVID-19 vaccine died from COVID-19; the Moderna and Janssen trials each had COVID-19 deaths in the placebo arm.
Preliminary data from the clinical trials suggest COVID-19 vaccination may also protect against asymptomatic infection.
In the Moderna trial, among people who had received a first dose, the number of asymptomatic people who tested positive for SARS-CoV-2 at their second-dose appointment was approximately two-thirds lower among vaccinees than among placebo recipients (0.1% and 0.3%, respectively).
Efficacy of Janssen COVID-19 vaccine against asymptomatic seroconversion was 74% in a subset of trial participants.
No trials have compared efficacy between any of the authorized vaccines in the same study at the same time.
All Phase 3 trials differed by calendar time and geography.
Vaccines were tested in settings with different background COVID-19 incidence and circulating variants.
Real-world vaccine effectiveness
Preliminary analyses from the United States and other countries demonstrate that a two-dose mRNA COVID-19 vaccination series is highly effective against SARS-CoV-2 infection (including both symptomatic and asymptomatic infections).
Table 1a. Effectiveness against SARS-CoV-2 infection and symptomatic disease
Country Population Vaccine Outcome Vaccine Effectiveness
United States14 General adult population Pfizer-BioNTech or Moderna SARS-CoV-2 infection 89%*
United States15 Healthcare workers, first responders, and other essential and frontline workers Pfizer-BioNTech or Moderna SARS-CoV-2 infection 90%**
United Kingdom16 Healthcare workers Pfizer-BioNTech SARS-CoV-2 infection 86%*
United Kingdom17 Adults aged ≥ 80 years, including those with multiple underlying conditions Pfizer-BioNTech Symptomatic disease 89%**
Israel18 General adult population Pfizer-BioNTech SARS-CoV-2 infection 92%*
General adult population Pfizer-BioNTech Symptomatic disease 94%*
General adult population Pfizer-BioNTech Hospitalization 87%*
General adult population Pfizer-BioNTech Severe disease 92%*
Israel19 General adult population Pfizer-BioNTech Symptomatic disease, severe/critical disease, death >97%**
Denmark20 Long term care facility residents Pfizer-BioNTech SARS-CoV-2 infection 64%*
Long term care facility residents Pfizer-BioNTech SARS-CoV-2 infection 90%*
* >7 days after second dose
** >14 days after second dose
In addition to the studies listed in Table 1a., further evidence of the impact of vaccination with Pfizer-BioNTech and Moderna COVID-19 vaccine has been demonstrated among healthcare workers with major reductions in SARS-CoV-2 infections among those receiving two doses of COVID-19 vaccine even in the setting of increasing community transmission.21-23
In contrast, data from nursing home residents demonstrate blunted antibody responses, which has important implications regarding the quality and durability of protection from COVID-19 vaccination in this population.24 Recent reports also suggest that people with solid organ transplants or patients on rituximab (an immunomodulating medication) have reduced antibody responses to the first dose of mRNA vaccination.25, 26 At this time, there are limited data on vaccine protection in people who are immunocompromised. People with immunocompromising conditions, including those taking immunosuppressive medications, should discuss the need for personal protective measures after vaccination with their healthcare provider.
Table 1b. Effectiveness against asymptomatic SARS-CoV-2 infection and transmission
Country Population Vaccine Outcome Vaccine effectiveness or risk reduction
United States27 General adult population Pfizer-BioNTech or Moderna Asymptomatic infection 80%*
United Kingdom (Scotland)28 Healthcare workers and household members Pfizer-BioNTech or AstraZeneca Household members: SARS-CoV-2 infection 54%**
Israel19 General adult population Pfizer-BioNTech Asymptomatic infection 94%**
* 0 days after second dose
** 14 days after second dose
Preliminary data from Israel suggest that people vaccinated with Pfizer-BioNTech COVID-19 vaccine who develop COVID-19 have a four-fold lower viral load than unvaccinated people.29 This observation may indicate reduced transmissibility, as viral load has been identified as a key driver of transmission.30
Vaccine performance against emerging SARS-CoV-2 variant strains
SARS-CoV-2 variants of concern (B.1.1.7 [first detected in the United Kingdom]; B.1.351 [first detected in South Africa]; P.1 [first detected in Japan/Brazil]; B.1.427 and B.1.429 [first detected in US-California]) have emerged with mutations that alter the receptor binding domain of the spike protein (notably the N501Y mutation occurring in B.1.1.7, B.1.351 and P.1 variants, as well as E484K and E417T/N mutations in B.1.351 and P.1).31 These mutations appear to confer greater resistance to neutralization by sera from people vaccinated with COVID-19 vaccines, raising concerns that these vaccines may have reduced effectiveness against COVID-19 illness due to strains with these mutations, particularly against the B.1.351 variant. Therefore, vaccine performance against emerging SARS-CoV-2 variants is an important consideration when evaluating the need for continued prevention measures in vaccinated people and will require continued monitoring.
Vaccine-induced neutralizing antibody activity
Sera from mRNA COVID-19 vaccine (both Pfizer-BioNTech and Moderna) recipients have generally demonstrated minimal to moderate reductions in antibody neutralization activity against a variety of mutations32-62; one study demonstrated poor neutralization activity for B.1.351.63 Across studies, the greatest reductions were observed for B.1.351, followed by P.1 and P.2 (a variant of interest first described in Brazil); reductions for B.1.1.7 and B.1.427/B.1.429 were minimal. The E484K mutation alone or in combination with other mutations in the receptor binding domain has been shown to account for the majority of reduction in vaccine-induced neutralizing antibody activity for the B.1351, P.1, and P.2 variants.35, 40, 42, 54, 56, 65 B.1.1.7 variants with E484K mutations, which have been detected in the United Kingdom, United States and other countries, have further reductions in neutralization above B.1.1.7 alone.35, 59, 66 For the Janssen viral vector COVID-19 vaccine, spike protein-specific antibody levels and seroresponse rates were similar between U.S. clinical trial participants and participants from Brazil and South Africa, where the viral variants were circulating.10 In the absence of a biological correlate of protection, it is difficult to predict how reduced neutralizing activity may affect COVID-19 vaccine effectiveness. However, across studies, antibody neutralizing activity of sera from vaccinated people was still generally higher than that observed for convalescent sera from people who have recovered from COVID-19.35, 38, 41-43, 48-51, 53, 54,56, 61
Efficacy and effectiveness
As described above, preliminary results from the United Kingdom demonstrate that vaccination with two doses of Pfizer-BioNTech COVID-19 vaccine was highly effective (85–86%) against SARS-CoV-2 infection and symptomatic COVID-19 during a period when B.1.1.7 was the predominant circulating strain.16, 17 Similarly, high Pfizer-BioNTech vaccine effectiveness (92%) against infection was observed in Israel in the context of multiple circulating strains, with the proportion of cases due to the B.1.1.7 variant increasing to 80% towards the end of the evaluation period.18 Preliminary data suggest that the Janssen COVID-19 vaccine may have reduced overall efficacy against the B.1.351 variant.10 In the United States, efficacy was 74%, and in Brazil (where ~69% of infections were due to P.2) efficacy was 66%, but in South Africa (~where 95% of infections were due to B.1.351) efficacy was 52%.10, 11 However, Janssen vaccine efficacy against severe or critical disease was high and similar across sites (73–82%).10
Impact of prevention measures in the context of vaccination
Individual and community-level prevention measures have been shown to help reduce the spread of SARS-CoV-2. These measures form the cornerstone for strategies to reduce viral transmission in the United States.1, 64-68 However, there are individual and societal costs related to physical distancing, quarantine, school and business closures, and other prevention measures.69-76
Modeling studies suggest that adherence to prevention measures, such as wearing masks and physical distancing, will continue to be important in the context of vaccine implementation.77-83 In one study, complete relaxation of prevention measures prior to adequate vaccination coverage resulted in essentially no reductions in SARS-CoV-2 infections.77 However, preliminary data suggest that increasing vaccination rates may allow for the phasing out of some prevention measures as coverage increases 83 [CDC unpublished]. Furthermore, there may be certain activities that can be performed after vaccination, such as nursing home visitationpdf iconexternal icon, as long as other prevention measures are maintained [CDC unpublished].
In summary, prevention measures will continue to be important for all people, regardless of vaccination status, especially during this period of vaccine deployment. However, as vaccination coverage increases, a balanced, stepwise approach to phasing out certain prevention measures in fully vaccinated people, ideally those that are the most disruptive to individuals and society, can be taken.
Population attitudes and behaviors towards vaccination and prevention measures
In surveys conducted since vaccination started in December 2020, approximately two-thirds of U.S. adults stated that they were at least somewhat likely to receive a COVID-19 vaccine (or had received one already).84-86 This suggests that continued efforts are needed to strengthen vaccine confidence and uptake, including addressing common concerns around COVID-19 vaccines (such as vaccine side effects, the speed of vaccine development, and mistrust of government), improving health equity by removing barriers to vaccine access, and using evidence-based approaches to improving uptake such as providing incentives for vaccination.85, 87 Leading reasons cited by U.S. adults for intending to be vaccinated include being able to return to more normal life, feeling safe around other people, and resuming activities like going to work or school.2, 88 Although it remains unknown which of these incentives would achieve the greatest increases in vaccination, information about activities that fully vaccinated people can safely undertake must be communicated in a clear and unambiguous fashion. Maintaining a requirement to continue all prevention measures after vaccination may disincentivize vaccine uptake. In a survey from January 2021, one in five people reported being less likely to get vaccinated if they heard that they will need to continue to wear a mask and practice social distancing even after getting vaccinated.2
A Harris Pollpdf iconexternal icon survey of U.S. adults, conducted in collaboration with CDC (March 12-14, 2021) following the initial release of CDC guidance for fully vaccinated people, suggests that most (83%) adults are aware of the new guidance and approximately half say they understand and plan to follow CDC’s guidance.89 Among unvaccinated respondents, almost half are motivated to get vaccinated by CDC’s efforts to roll back some mitigation measures for fully vaccinated people, stating that they’d be more likely to get vaccinated knowing that fully vaccinated people could now resume small private gatherings with other fully vaccinated people (47%) or with unvaccinated family and friends of the same household (40%), and would no longer need to get tested or quarantine following a known exposure to COVID-19 (42%).89 Additional stepped-down measures could further motivate vaccination, with nearly half saying they would be more likely to get vaccinated if they could also resume domestic travel without the need for testing or quarantine (47%), visit indoor spaces and businesses without a mask (48%), and return to their office or campus (41%).89
In summary, relaxing certain prevention measures for fully vaccinated people may be a powerful motivator for vaccination, and thus should be an important goal of the U.S. vaccination program.
Rationale and evidence for travel associated public health recommendations for fully vaccinated travelers
Background
Individuals may be at risk for exposure to SARS-CoV-2 before, during, and after travel. This could result in spread of the virus throughout the travel journey, at destinations, or upon returning home. Comprehensive, layered prevention measures before, during, and after travel are critical strategies to reduce the burden of travel-associated spread of SARS-CoV-2. To reduce this risk, CDC has recommended that all travelers be tested for the virus 1–3 days before departure (required for air passengers traveling to the United States from a foreign country unless they can present documentation of recovery from COVID-19 in the previous 3 months), combined with a second test 3–5 days after travel and staying home to self-quarantine for a full 7 days after travel if the post-travel test result is negative. If post-travel testing is not done, the recommendation is to extend self-quarantine to 10 days after travel.90, 91
Public health decisions regarding relaxing travel-related recommendations for those who are fully vaccinated should take into account all available evidence, including vaccine effectiveness for preventing SARS-CoV-2 infection and transmission. Individual and societal factors are also important when evaluating the benefits and potential harms of prevention measures among vaccinated travelers. Testing and quarantine are resource-intensive prevention measures that impose a burden on public health resources, individual travelers, workplaces, and communities. In circumstances where SARS-CoV-2 incidence is low, many tests may be needed to identify a small number of infected travelers. Many uninfected travelers may also undergo unnecessary quarantine, which could result in an undue burden of missed work (including for essential workers) or school. In circumstances where the incidence and the risk or consequences of introduction are high, testing and self-quarantine can substantially reduce transmission.
Impact of prevention measures for travelers in the context of vaccination
To assess the feasibility of eliminating post-travel testing and self-quarantine recommendations for vaccinated travelers, data from studies that evaluated vaccine effectiveness for infection prevention (regardless of symptoms) (Table 1a) were reviewed. To estimate the impact of vaccination combined with other measures, a range of vaccine effectiveness was used in models previously developed to assess testing and quarantine prevention measures.92 There are currently several vaccines available globally with varying efficacy rates and limited data regarding effectiveness, including against circulating variants; estimates were based solely on data available from the three vaccines authorized in the United States.
Preliminary data from Israel suggest that people vaccinated with the Pfizer-BioNTech COVID-19 vaccine who develop COVID-19 have a four-fold lower viral load than unvaccinated people.29 This observation may indicate reduced transmissibility, as viral load has been identified as a key driver of transmission.29, 30
Vaccinated travelers may thus be less likely to be infected (VES: susceptibility) and to transmit to others if infected (VEI: infection). Early estimates of VES for the Pfizer-BioNTech vaccine are on the order of 60–92%. Therefore, a range of combined VES and VEI estimates from 60–90% for vaccinated travelers was assessed. For example, with a combined VE of 60%, a traveler, who if unvaccinated would be infected and infectious 100% of the time, was assumed to be infected and infectious 40% of the time and a combined VE of 90%, the traveler was assumed to be infected and infectious 10% of the time. In all scenarios, it was assumed that travelers were tested by real time reverse transcription polymerase chain reaction (RT-PCR) or antigen test 1–3 days before departure. Symptom monitoring was not included in any scenario and, when included in the model, all travelers were assumed to adhere to a post-travel self-quarantine for 7 days. In some scenarios, a post-arrival test at day 3–5 was added.
With a 90% effective vaccine, pre-travel testing, post-travel testing, and 7-day self-quarantine provide minimal additional benefit. When adhered to, a pre-travel test plus a 7-day post-arrival self-quarantine for unvaccinated travelers is >30% more effective at reducing risk compared to travelers vaccinated with a 60% effective vaccine (Table 2).
Population-level vaccination coverage and travel
Two other factors may contribute to risk associated with travel: (1) the risk of infection at the origin location of the traveler and (2) the risk of transmission at the destination. Both of these factors are influenced by vaccination coverage rates. With increasing vaccination coverage in populations at origins and destinations, the infection and transmission risks for the traveler would be reduced (Figure 1). This provides additional risk reductions, proportional to coverage and effectiveness, for both vaccinated and unvaccinated travelers.
There are many factors that are unknown for international travel such as effectiveness of non–FDA-authorized vaccines, vaccination coverage in populations at origins and destinations, circulating SARS-CoV-2 variants, and vaccine effectiveness against these variants. Therefore, pre- and post-travel testing may provide an additional layer of risk reduction for international travelers without the additional burden of self-quarantine.
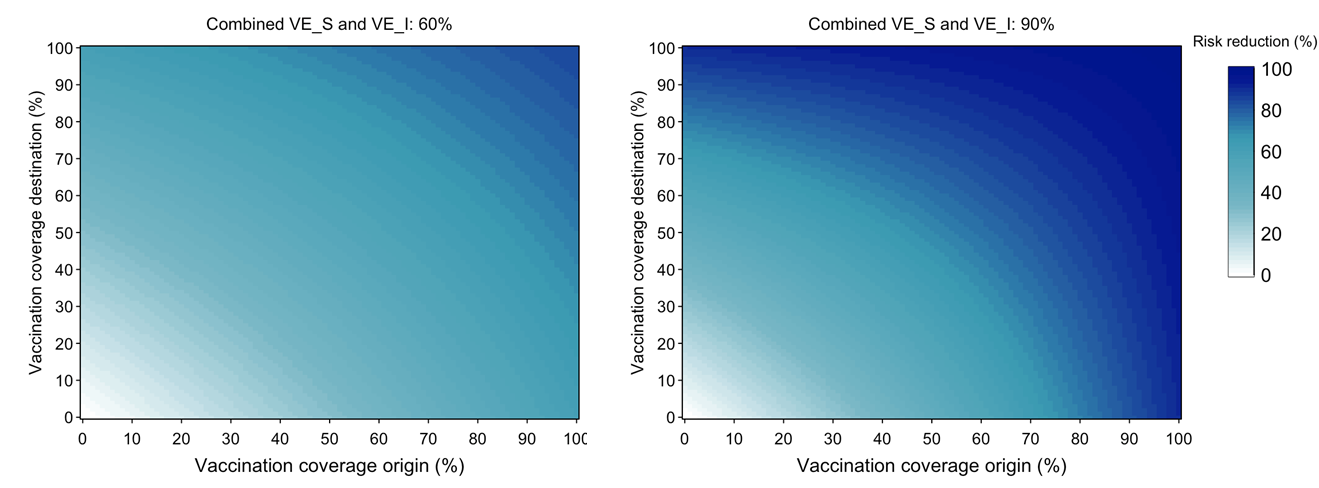
Figure 1: Impact of origin and destination vaccine coverage and vaccine efficacy (combined VES and VEI) of 60% and 90% on introduction risk reduction
With 60% combined VE and 100% of people in the origin location vaccinated and 0% in the destination location, there is a 60% reduction in the risk of introduction by an exposed traveler at the destination compared to if there were no vaccination in either population. However, if 50% of the destination population is also vaccinated, that reduction increases to 72%. If both populations have full vaccination coverage, the reduction is 84%. The absolute risk (i.e., considering prevalence of infection not just vaccination) is lower as transmission is unlikely to be widespread in the origin location with 100% vaccine coverage. With 90% combined VE, substantial reductions occur at lower vaccination coverage proportions.
Table 2: Model results examining percent risk reduction relative to baseline where unvaccinated individual is exposed at origin and takes no precautions
Post-travel risk reduction
Vaccine effectiveness against infection Pre-travel testa 7-day quarantine Post-travel test day 3–5 Median Min Max
No vaccine 0% 0% 0%
No vaccine check light icon 13.6% 4.5% 35.5%
No vaccine check light icon check light icon 43.3% 19.7% 66.0%
No vaccine check light icon check light icon 92.4% 66.2% 94.7%
No vaccine check light icon check light icon check light icon 97.1% 85.8% 98.9%
60% 60.0% 60.0% 60.0%
60% check light icon 65.4% 61.8% 74.2%
60% check light icon check light icon 77.3% 67.9% 86.4%
60% check light icon check light icon 97.0% 86.5% 97.9%
60% check light icon check light icon check light icon 98.9% 94.3% 99.6%
70% 70.0% 70.0% 70.0%
70% check light icon 74.1% 71.4% 80.6%
70% check light icon check light icon 83.0% 75.9% 89.8%
70% check light icon check light icon 97.7% 89.9% 98.4%
70% check light icon check light icon check light icon 99.1% 95.7% 99.7%
80% 80.0% 80.0% 80.0%
80% check light icon 82.7% 80.9% 87.1%
80% check light icon check light icon 88.7% 83.9% 93.2%
80% check light icon check light icon 98.5% 93.2% 98.9%
80% check light icon check light icon check light icon 99.4% 97.2% 99.8%
90% 90.0% 90.0% 90.0%
90% check light icon 91.4% 90.5% 93.5%
90% check light icon check light icon 94.3% 92.0% 96.6%
90% check light icon check light icon 99.2% 96.6% 99.5%
90% check light icon check light icon check light icon 99.7% 98.6% 99.9%
a Pre-travel test 1–3 days before departure as required for all people entering the United States [91] and recommended for other travelers. It is assumed that if a pre- or post-travel test is positive, the traveler does not travel or isolates, respectively, until recovered.
These findings suggest that any travel-associated transmission risk is likely to be substantially reduced among those fully vaccinated with an effective vaccine. The risks of SARS-CoV-2 infection in fully vaccinated travelers cannot be completely eliminated in the setting of continued widespread transmission. Travel presents opportunities for onward viral transmission due to mixing of individuals throughout the travel journey. It is not fully known how vaccination will impact infection and forward transmission. However, the evidence suggests that with an effective vaccine and increasing vaccination coverage, the burden of testing and quarantine is much higher than any marginal additional risk reduction provided for the fully vaccinated traveler. Taking into consideration the factors outlined above, CDC has updated both domestic and international travel guidance.
However, with inconsistent and varying vaccination coverage rates from country to country, and because vaccine effectiveness data were limited to those receiving a subset of globally available vaccines, the analyses described here should not be extrapolated to other vaccines. Global circulation of SARS-CoV-2 variants and vaccine performance against emerging variants are important considerations when evaluating the need for continued prevention measures in vaccinated people and will require continued monitoring. As such, CDC will continue to require a negative SARS-CoV-2 test result or documentation of recovery from COVID-19 for all air passengers departing for the United States.91 CDC continues to review available data and will update travel guidance accordingly.
Conclusions
COVID-19 vaccines currently authorized in the United States have been shown to be efficacious and effective against SARS-CoV-2 infections, including asymptomatic infection, symptomatic disease, severe disease, and death. These findings, along with the potential for reduced viral load in vaccinated people who develop COVID-19, suggest that any associated transmission risk is likely to be substantially reduced in vaccinated people. While vaccine efficacy against emerging SARS-CoV-2 variants remains under investigation, preliminary evidence suggests that the COVID-19 vaccines presently authorized in the United States will likely be effective against emerging variants, though reduced antibody neutralization and efficacy has been observed for the B.1.351 variant.
Evidence suggests the U.S. COVID-19 vaccination program has the potential to substantially reduce the burden of disease in the United States by preventing illness in fully vaccinated people and interrupting chains of transmission. The risks of SARS-CoV-2 infection in fully vaccinated people cannot be completely eliminated in the setting of continued widespread community transmission of the virus. Vaccinated people could potentially still become infected and spread the virus to others. However, the benefits of avoiding disruptions such as unnecessary quarantine and social isolation may outweigh these potential residual risks. A balanced approach to phasing out certain prevention measures may be a powerful motivator for vaccination, and thus should be an important goal of the U.S. vaccination program.
References
Note: Preprints have not been peer-reviewed. They should not be regarded as conclusive, guide clinical practice/health-related behavior, or be reported in news media as established information.
Honein MA, Christie A, Rose DA, Brooks JT, Meaney-Delman D, Cohn A, et al. Summary of Guidance for Public Health Strategies to Address High Levels of Community Transmission of SARS-CoV-2 and Related Deaths, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69(49):1860-7.
Kaiser Family Foundation. KFF COVID-19 Vaccine Monitor: January 20212021; (March 24, 2021). Available from: https://www.kff.org/report-section/kff-covid-19-vaccine-monitor-january-2021-vaccine-hesitancy/external icon.
Lee G, Carr W, Group AE-BRW, Group AEBRW. Updated Framework for Development of Evidence-Based Recommendations by the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018;67(45):1271-2.
Corbett KS, Flynn B, Foulds KE, Francica JR, Boyoglu-Barnum S, Werner AP, et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N Engl J Med. 2020;383(16):1544-55.
Mercado NB, Zahn R, Wegmann F, Loos C, Chandrashekar A, Yu J, et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586(7830):583-8.
Vogel AB, Kanevsky I, Che Y, Swanson KA, Muik A, Vormehr M, et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021.
Food and Drug Administration. Pfizer-BioNTech COVID-19 Vaccine. Vaccines and Related Biological Products Advisory Committee Briefing Document – Sponsor. https://www.fda.gov/media/144246/downloadexternal icon.
Food and Drug Administration. Moderna COVID-19 Vaccine. Vaccines and Related Biological Products Advisory Committee December 17, 2020 Meeting Briefing Document- Sponsor. https://www.fda.gov/media/144452/downloadexternal icon.
Food and Drug Administration. Moderna COVID-19 Vaccine. Vaccines and Related Biological Products Advisory Committee December 17, 2020 Meeting Briefing Document Addendum- Sponsor. https://www.fda.gov/media/144453/downloadexternal icon.
Food and Drug Administration. Janssen COVID-19 Vaccine. Vaccines and Related Biological Products Advisory Committee February 26, 2021 Meeting Briefing Document – Sponsor. https://www.fda.gov/media/146219/downloadexternal icon.
Food and Drug Administration. Janssen COVID-19 Vaccine. Vaccines and Related Biological Products Advisory Committee February 26, 2021 Meeting Briefing Document Addendum – Sponsor. https://www.fda.gov/media/146218/downloadexternal icon.
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403-16.
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603-15.
Pawlowski C LP, Puranik A, et. al. FDA-authorized COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.02.15.21251623v1.full.pdfpdf iconexternal icon.
Thompson MG BJ, Naleway AL, et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers — Eight U.S. Locations, December 2020–March 2021. MMWR Morb Mortal Wkly Rep. 2021;ePub: 29 March 2021. DOI: http://dx.doi.org/10.15585/mmwr.mm7013e3external icon.
Hall A FS, Sae A, et. al. . Effectiveness of BNT162b2 mRNA Vaccine Against Infection and COVID-19 Vaccine Coverage in Healthcare Workers in England, Multicentre Prospective Cohort Study (the SIREN Study). . Lancet (preprint). 2021;https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3790399(Marchexternal icon 4, 2021).
Bernal J AN, Gower C, et. al. . Early Effectiveness of COVID-19 Vaccination with BNT162b2 mRNA Vaccine and ChAdOx1 Adenovirus Vector Vaccine on Symptomatic Disease, Hospitalisations and Mortality in Older Adults in England. medRxiv. 2021;https://khub.net/documents/135939561/430986542/Early+effectiveness+of+COVID+vaccines.pdf/ffd7161c-b255-8e88-c2dc-88979fc2cc1b?t=1614617945615external icon.
Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021.
Real-World Evidence Confirms High Effectiveness of Pfizer-BioNTech COVID-19 Vaccine and Profound Public Health Impact of Vaccination One Year After Pandemic Declared [press release]. March 11, 2021 2021.
Mostsen-Helms I EH, Nielsen J, et. al. Vaccine effectiveness after 1st and 2nd dose of the BNT162b2 mRNA Covid-19 Vaccine in long-term care facility residents and healthcare workers – a Danish cohort study medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.03.08.21252200v1.full.pdf(Marchexternal icon 24, 2021).
Benenson S, Oster Y, Cohen MJ, Nir-Paz R. BNT162b2 mRNA Covid-19 Vaccine Effectiveness among Health Care Workers. N Engl J Med. 2021.
Daniel W, Nivet M, Warner J, Podolsky DK. Early Evidence of the Effect of SARS-CoV-2 Vaccine at One Medical Center. N Engl J Med. 2021.
Keehner J, Horton LE, Pfeffer MA, Longhurst CA, Schooley RT, Currier JS, et al. SARS-CoV-2 Infection after Vaccination in Health Care Workers in California. N Engl J Med. 2021.
Canaday D CL, Oyebanji O, et al. Reduced BNT162b2 mRNA vaccine response in SARS-CoV-2 naive nursing home residents. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.03.19.21253920v1external icon.
Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Immunogenicity of a Single Dose of SARS-CoV-2 Messenger RNA Vaccine in Solid Organ Transplant Recipients. JAMA. 2021.
Boyarsky BJ, Ruddy JA, Connolly CM, Ou MT, Werbel WA, Garonzik-Wang JM, et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021.
Tande AJ, Pollock BD, Shah ND, Farrugia G, Virk A, Swift M, et al. Impact of the COVID-19 Vaccine on Asymptomatic Infection Among Patients Undergoing Pre-Procedural COVID-19 Molecular Screening. Clin Infect Dis. 2021.
Shah A GC, Bishop J, et al. Effect of vaccination on transmission of COVID-19: an observational study in healthcare workers and their households. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.03.11.21253275v1external icon.
Levine-Tiefenbrun M YI, Katz R, et al. . Decreased SARS-CoV-2 viral load following vaccination. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.02.06.21251283v1.full.pdfpdf iconexternal icon.
Marks M, Millat-Martinez P, Ouchi D, Roberts CH, Alemany A, Corbacho-Monne M, et al. Transmission of COVID-19 in 282 clusters in Catalonia, Spain: a cohort study. Lancet Infect Dis. 2021.
Centers for Disease Control and Prevention. SARS-CoV-2 Variant Classifications and Definitions [Available from: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/variant-surveillance/variant-info.html#Concern.
Annavajhala MK, Mohri H, Zucker JE, Sheng Z, Wang P, Gomez-Simmonds A, et al. A Novel SARS-CoV-2 Variant of Concern, B.1.526, Identified in New York. medRxiv. 2021;https://www.ncbi.nlm.nih.gov/pubmed/33655278external icon.
Becker M DA, Junker D, et al. Immune response to SARS-CoV-2 variants of concern in vaccinated individuals. medRxiv. 2021;https://doi.org/10.1101/2021.03.08.21252958external icon.
Chen RE, Zhang X, Case JB, Winkler ES, Liu Y, VanBlargan LA, et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med. 2021.
Collier DA, De Marco A, Ferreira I, Meng B, Datir R, Walls AC, et al. Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature. 2021.
Dejnirattisai W. ZD, Supasa P., et al. Antibody evasion by the Brazilian P.1 strain of SARS-CoV-2. bioRxiv. 2021;https://doi.org/10.1101/2021.03.12.435194external icon.
Deng X, Garcia-Knight MA, Khalid MM, Servellita V, Wang C, Morris MK, et al. Transmission, infectivity, and antibody neutralization of an emerging SARS-CoV-2 variant in California carrying a L452R spike protein mutation. medRxiv. 2021;https://www.ncbi.nlm.nih.gov/pubmed/33758899external icon.
Edara VV, Hudson WH, Xie X, Ahmed R, Suthar MS. Neutralizing Antibodies Against SARS-CoV-2 Variants After Infection and Vaccination. JAMA. 2021.
Emary K GT, Aley P, et al. . Efficacy of ChAdOx1 nCoV-19 (AZD1222) Vaccine Against SARS-CoV-2 VOC 202012/01 (B.1.1.7). Lancet (preprint). 2021;https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3779160external icon.
Garcia-Beltran WF, Lam EC, St Denis K, Nitido AD, Garcia ZH, Hauser BM, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021.
Hoffmann M AP, Seidel A, et al. SARS-CoV-2 variants B.1.351 and B.1.1.248: Escape from therapeutic antibodies and antibodies induced by infection and vaccination. bioRxiv. 2021;https://www.biorxiv.org/content/10.1101/2021.02.11.430787v1external icon.
Jangra S, Ye C, Rathnasinghe R, Stadlbauer D, Krammer F, Simon V, et al. The E484K mutation in the SARS-CoV-2 spike protein reduces but does not abolish neutralizing activity of human convalescent and post-vaccination sera. medRxiv. 2021;https://www.ncbi.nlm.nih.gov/pubmed/33532796external icon.
Kuzmina A KY, Voloshin O, et al. SARS CoV-2 Escape Variants Exhibit Differential Infectivity and Neutralization Sensitivity to Convalescent or Post-Vaccination Sera. Cell Host & Microbe. 2021.
Liu Y, Liu J, Xia H, Zhang X, Fontes-Garfias CR, Swanson KA, et al. Neutralizing Activity of BNT162b2-Elicited Serum. N Engl J Med. 2021.
Marot S MI, Jary A, et al. Neutralization heterogeneity of United Kingdom and South-African SARS-CoV-2 variants in BNT162b2-vaccinated or convalescent COVID-19 healthcare workers. bioRxiv. 2021;https://doi.org/10.1101/2021.03.05.434089external icon.
Muik A, Wallisch AK, Sanger B, Swanson KA, Muhl J, Chen W, et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science. 2021;371(6534):1152-3.
Planas D BT, Grzelak L, et al. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. bioRxiv. 2021;https://www.biorxiv.org/content/10.1101/2021.02.12.430472v1external icon.
Rathnasinghe R, Jangra S, Cupic A, Martinez-Romero C, Mulder LCF, Kehrer T, et al. The N501Y mutation in SARS-CoV-2 spike leads to morbidity in obese and aged mice and is neutralized by convalescent and post-vaccination human sera. medRxiv. 2021;https://www.ncbi.nlm.nih.gov/pubmed/33501468external icon.
Sahin U MA, Vogler I, et al. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2020.12.09.20245175v1external icon.
Shen X, Tang H, McDanal C, Wagh K, Fischer W, Theiler J, et al. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe. 2021.
Skelly D HA, Gilbert-Jaramillo J, et al. Vaccine-induced immunity provides more robust heterotypic immunity than natural infection to emerging SARS-CoV-2 variants of concern. Research Square. 2021;https://www.researchsquare.com/article/rs-226857/v1external icon.
Stamatatos L, Czartoski J, Wan YH, Homad LJ, Rubin V, Glantz H, et al. A single mRNA immunization boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. medRxiv. 2021;https://www.ncbi.nlm.nih.gov/pubmed/33758873external icon.
Supasa P, Zhou D, Dejnirattisai W, Liu C, Mentzer AJ, Ginn HM, et al. Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell. 2021.
Tada T, Dcosta BM, Samanovic-Golden M, Herati RS, Cornelius A, Mulligan MJ, et al. Neutralization of viruses with European, South African, and United States SARS-CoV-2 variant spike proteins by convalescent sera and BNT162b2 mRNA vaccine-elicited antibodies. bioRxiv. 2021;https://www.ncbi.nlm.nih.gov/pubmed/33564768external icon.
Trinite B PE, Marfil S, et al. Previous SARS-CoV-2 infection increases B.1.1.7 cross-neutralization by vaccinated individuals. bioRxiv. 2021;https://doi.org/10.1101/2021.03.05.433800external icon.
Wang P, Nair MS, Liu L, Iketani S, Luo Y, Guo Y, et al. Antibody Resistance of SARS-CoV-2 Variants B.1.351 and B.1.1.7. Nature. 2021.
Wang P. WM, Yu J., et al. . BNT162b vaccines are immunogenic and protect non-human primates against SARS-CoV-2. bioRxiv. 2021;https://www.biorxiv.org/content/10.1101/2020.12.11.421008v1.fullexternal icon.
Wang Z, Schmidt F, Weisblum Y, Muecksch F, Barnes CO, Finkin S, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021.
Wu K, Werner AP, Koch M, Choi A, Narayanan E, Stewart-Jones GBE, et al. Serum Neutralizing Activity Elicited by mRNA-1273 Vaccine. N Engl J Med. 2021.
Xie X, Liu Y, Liu J, Zhang X, Zou J, Fontes-Garfias CR, et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat Med. 2021.
Zhou D, Dejnirattisai W, Supasa P, Liu C, Mentzer AJ, Ginn HM, et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021.
Zhou H DB, Samanovic M, et al. . B.1.526 SARS-CoV-2 variants identified in New York City are neutralized by vaccine-elicited and therapeutic monoclonal antibodies. bioRxiv. 2021;https://www.biorxiv.org/content/10.1101/2021.03.24.436620v1.full.pdfpdf iconexternal icon.
Madhi SA, Baillie V, Cutland CL, Voysey M, Koen AL, Fairlie L, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med. 2021.
Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schunemann HJ, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395(10242):1973-87.
Gallaway MS, Rigler J, Robinson S, Herrick K, Livar E, Komatsu KK, et al. Trends in COVID-19 Incidence After Implementation of Mitigation Measures – Arizona, January 22-August 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(40):1460-3.
Haug N, Geyrhofer L, Londei A, Dervic E, Desvars-Larrive A, Loreto V, et al. Ranking the effectiveness of worldwide COVID-19 government interventions. Nat Hum Behav. 2020;4(12):1303-12.
Kanu FA, Smith EE, Offutt-Powell T, Hong R, Delaware Case I, Contact Tracing T, et al. Declines in SARS-CoV-2 Transmission, Hospitalizations, and Mortality After Implementation of Mitigation Measures- Delaware, March-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(45):1691-4.
Kucharski AJ, Klepac P, Conlan AJK, Kissler SM, Tang ML, Fry H, et al. Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS-CoV-2 in different settings: a mathematical modelling study. Lancet Infect Dis. 2020;20(10):1151-60.
Alexander A RJ, Smetters K, et al. Epidemiological and Economic Effects of Lockdown. Brookings Papers on Economic Activity. 2020;https://www.brookings.edu/wp-content/uploads/2020/09/Arnon-et-al-conference-draft.pdfpdf iconexternal icon.
Boserup B, McKenney M, Elkbuli A. Alarming trends in US domestic violence during the COVID-19 pandemic. Am J Emerg Med. 2020;38(12):2753-5.
Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912-20.
Czeisler ME, Lane RI, Petrosky E, Wiley JF, Christensen A, Njai R, et al. Mental Health, Substance Use, and Suicidal Ideation During the COVID-19 Pandemic – United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049-57.
Holland KM, Jones C, Vivolo-Kantor AM, Idaikkadar N, Zwald M, Hoots B, et al. Trends in US Emergency Department Visits for Mental Health, Overdose, and Violence Outcomes Before and During the COVID-19 Pandemic. JAMA Psychiatry. 2021.
McGinty EE, Presskreischer R, Han H, Barry CL. Psychological Distress and Loneliness Reported by US Adults in 2018 and April 2020. JAMA. 2020;324(1):93-4.
Orben A, Tomova L, Blakemore SJ. The effects of social deprivation on adolescent development and mental health. Lancet Child Adolesc Health. 2020;4(8):634-40.
UNESCO. Adverse consequences of school closures.
Galanti M PS, Yamana TK, et al. The importance of continued non-pharmaceutical interventions during the upcoming SARS-COV-2 vaccination campaign. medRxiv. 2020;https://www.medrxiv.org/content/10.1101/2020.12.23.20248784v1external icon.
Gozzi N BP, Perra N, et al. The importance of non-pharmaceutical interventions during the COVID-19 vaccine rollout. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.01.09.21249480v1.full.pdfpdf iconexternal icon.
Gumel A IE, Ngonghala C, et al. Towards achieving a vaccine-derived herd immunity threshold for COVID-19 in the U.S. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2020.12.11.20247916v3external icon.
Iboi EA, Ngonghala CN, Gumel AB. Will an imperfect vaccine curtail the COVID-19 pandemic in the U.S.? Infect Dis Model. 2020;5:510-24.
Li J GP. Returning to a normal life via COVID-19 vaccines in the USA: a large-scale agent-based simulation study. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.01.31.21250872v1external icon.
Love J KL, Angulo F, et al. Continued need for non-pharmaceutical interventions after COVID-19 vaccination in long-termcare facilities. medRxiv. 2021;https://www.medrxiv.org/content/10.1101/2021.01.06.21249339v1.full.pdfpdf iconexternal icon.
Tang B LP, Yang J. The challenges of the coming mass vaccination and exit strategy in prevention and control of COVID-19, a modelling study. medRxiv. 2020;https://www.medrxiv.org/content/10.1101/2020.12.18.20248478v1external icon.
Axios/Ipsos Poll – Wave 40. [Available from: https://www.ipsos.com/sites/default/files/ct/news/documents/2021-03/topline-axios-ipsos-coronavirus-index-w40.pdfpdf iconexternal icon.
Nguyen KH, Srivastav A, Razzaghi H, Williams W, Lindley MC, Jorgensen C, et al. COVID-19 Vaccination Intent, Perceptions, and Reasons for Not Vaccinating Among Groups Prioritized for Early Vaccination – United States, September and December 2020. MMWR Morb Mortal Wkly Rep. 2021;70(6):217-22.
Szilagyi PG, Thomas K, Shah MD, Vizueta N, Cui Y, Vangala S, et al. National Trends in the US Public’s Likelihood of Getting a COVID-19 Vaccine-April 1 to December 8, 2020. JAMA. 2020.
The Community Guide. Increasing Appropriate Vaccination: Client or Family Incentive Rewards [Available from: https://www.thecommunityguide.org/sites/default/files/assets/Vaccination-Incentive-Rewards.pdfpdf iconexternal icon.
AP/NORC. Many remain doubtful about getting COVID-19 vaccine [Available from: https://apnorc.org/projects/many-remain-doubtful-about-getting-covid-19-vaccine/external icon.
Poll H. Harris Poll COVID-19 Survey Wave 55 2021 [March 24, 2021]. Available from: https://theharrispoll.com/wp-content/uploads/2021/03/Wave-55-Data-Tabs.pdfpdf iconexternal icon.
Centers for Disease Control and Prevention. Travel During COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/travelers/travel-during-covid19.html.
Centers for Disease Control and Prevention. Requirement for Negative Pre-Departure COVID-19 Test Result or Documentation of Recovery from COVID-19 for All Airline or Other Aircraft Passengers Arriving Into the United States from Any Foreign Country. https://www.cdc.gov/quarantine/pdf/Global-Airline-Testing-Order-RPWSigned-Encrypted-p.pdf2021.
Johansson M WH, Paul P, et al. Reducing travel-related SARS-CoV-2 transmission with layered mitigation measures: Symptom monitoring, quarantine, and testing. medRxiv. 2020;https://doi.org/10.1101/2020.11.23.20237412external icon.
Sem comentários:
Enviar um comentário